Anal Cancer Screening
A reference guide for Physicians on screening gay and bisexual men for anal cancer.
Anal cancer is more prevalent among gay and bisexual men compared to heterosexual men and, like cervical cancer in women, is associated with human papillomavirus (HPV) infection.
HPV is a sexually transmitted virus that can cause genital warts and, in men, is associated with squamous cell carcinomas of the anus, penis and oropharynx. In fact, HPV is responsible for 80-90% of all anal cancers (4). Studies have shown that gay and other men who have sex with men are about 20-times more likely and HIV+ men who have sex with men are about 80-100 times more likely to develop anal cancer compared to heterosexual men (3). It is important then to inform and screen gay and bisexual men for anal cancer.
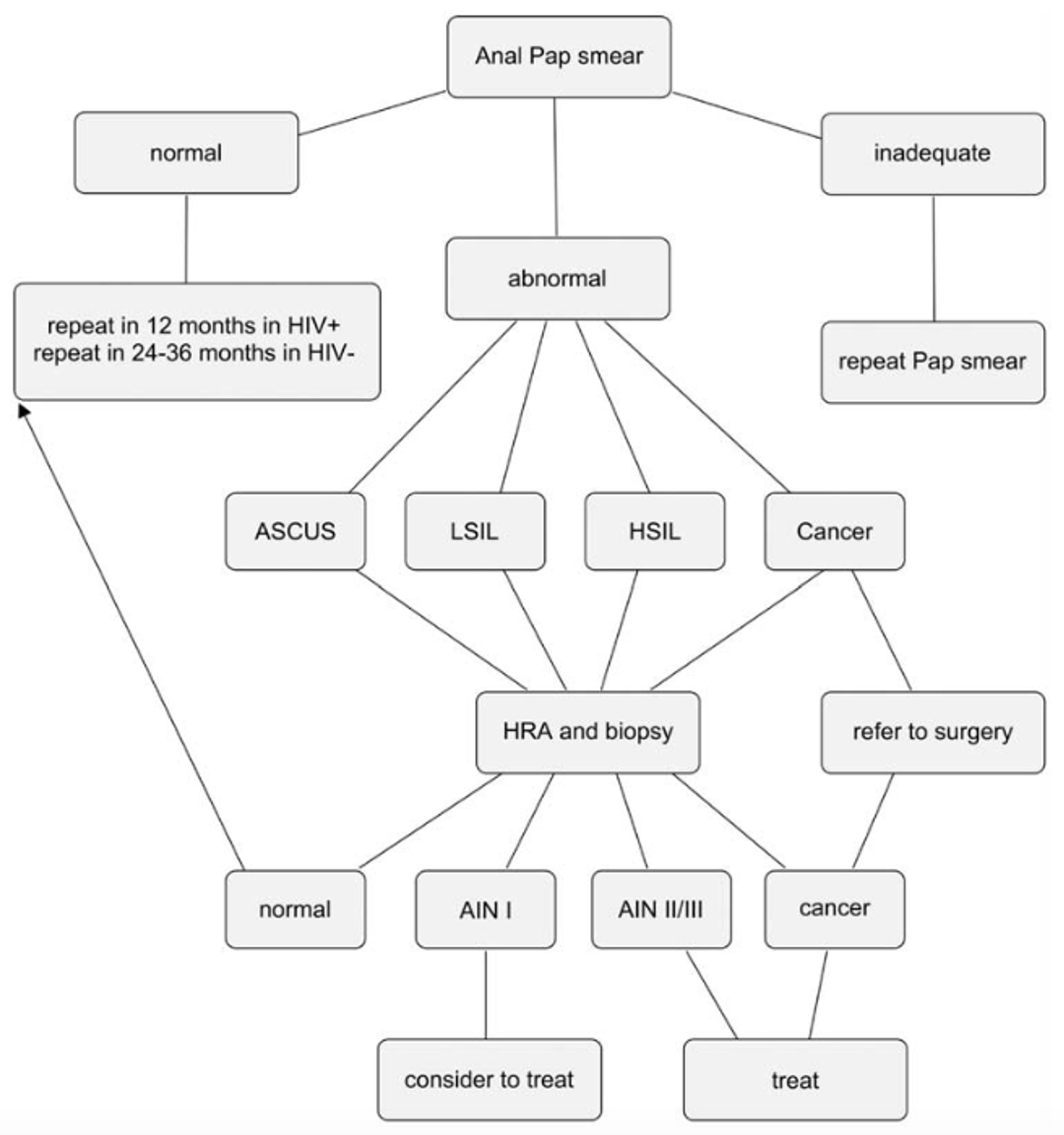
Currently, there are no guidelines for anal cancer screening. However, early detection has been shown to decrease morbidity and increase survival (2). The approach to screening depends on the availability of high resolution anoscopy (HRA) in your area. HRA is a technique similar to colposcopy where the anal canal and specifically the squamocolumnar junction between the anus and the rectum is examined under magnification using a colposcope.
If referral for HRA is NOT available:
All HIV-positive men and HIV-negative men beginning at age 40 should undergo an annual anal physical exam.
First, palpate the inguinal areas for nodes. Then, visually inspect the perianal region looking for external hemorrhoids, fissures, skin tags, and warts. Next, perform a Digital Anal-Rectal Examination (DARE) by placing a lubricated finger in the anus and palpating the full circumference of the anal wall feeling for masses, nodules and tenderness (5).
If referral for HRA is available:
Howard Brown Health has published the following screening guidelines (5):
| HIV Status | Age | Test | Frequency |
| HIV Positive | Under 30 yo | DARE (Digital anal-rectal exam) | annually |
| HIV Positive | 30 yo and above | Anal Pap and DARE
| annually |
| HIV Negative | 40 yo and above | Anal Pap and DARE
| annually |
The purpose of an anal pap test is to collect cells from the walls of the anal canal. Anal cancer occurs mainly in two places, at the anal-rectum (squamocolumnar) junction and in the perianal region just outside the anal opening.
Patients should avoid anal intercourse, using toys, douches and enemas 24 hours before an anal pap test. And, the anal pap should be performed before a digital rectal exam because lubrication may alter results.
Materials required:
- Dacron swab
- Cytology fixative solution
- Scissors
Collection instructions:
- Have the patient in the lateral recumbent position with knees bent
- Moisten the Dacron swab with tap water
- Insert the swab about 5 cm into the anal canal
- Move the swab in a circular motion pressing against the walls of the anal canal. Do this 10-12 times as the swab is slowly withdrawn.
- Place the tip of the swab in the Cytology solution. Cut off the tip leaving it in the solution and discard the handle of the swab.
- Screw the cap on the specimen container and tighten.
HPV Vaccine (Gardasil-9)
The best way to prevent anal cancer is to prevent HPV infection. The HPV vaccine is both safe and effective. The Gardasil-9 vaccine protects against HPV types 6, 11, 16, 18, 31, 33, 45, 52, and 58 which includes those causing genital warts and the most prevalent HPV types causing cancer. The Canadian National Advisory Committee on Immunizations states Gardasil should be considered for all men who have sex with men regardless of age. In Ontario, Gardasil-9 is recommended and publicly funded for gay and bisexual men under 27 at no cost. For men 27 and over, Gardasil-9 is available for a fee or may be covered by patient’s health insurance. The Canadian Cancer Society estimates that it costs around $600 for three doses of the Gardasil-9 vaccine.